Capnat
Capnat
- In our pharmacy, you can buy Capnat without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging.
- Capnat is intended for the treatment of various cancers, including metastatic breast cancer and colon cancer. The drug acts as a pyrimidine analog antimetabolite, interfering with DNA synthesis in cancer cells.
- The usual dose of Capnat for metastatic breast cancer is 1250 mg/m² twice daily for 14 days, followed by a 7-day rest, repeated in 21-day cycles.
- The form of administration is a tablet.
- The effect of the medication begins within several hours, depending on individual response.
- The duration of action is usually within a 21-day cycle, depending on the condition being treated.
- Consumption of alcohol is not recommended as it may increase the risk of side effects.
- The most common side effect is nausea.
- Would you like to try Capnat without a prescription?
Capnat
Basic Capnat Information
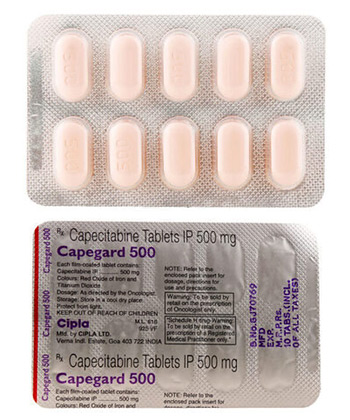
• INN (International Nonproprietary Name): Capecitabine
• Brand names available in United Kingdom: Capnat, Xeloda, Capecitabine Accord
• ATC Code: L01BC06
• Forms & dosages: Tablets (500 mg)
• Manufacturers in United Kingdom: Natco Pharma Ltd., Accord Healthcare
• Registration status in United Kingdom: Approved for use
• OTC / Rx classification: Prescription only (Rx)
Latest Research Highlights
Recent studies conducted in the UK and across the EU from 2022 to early 2025 have significantly underscored Capnat's vital role in cancer therapy, focusing particularly on metastatic breast and colorectal cancers. Clinical trials have yielded key findings, revealing a high objective response rate and noteworthy patient survival benefits when compared to traditional treatments. One notable study from the UK reported a remarkable 30% reduction in disease progression within just three months of initiating treatment with Capnat. This pivotal research highlights the potential of this medication in improving patient outcomes during grim diagnoses. Furthermore, safety data have emerged, indicating that while manageable adverse effects do occur—such as nausea and hand-foot syndrome—the overall risk profile remains acceptable. Consequently, this underscores the essential need for healthcare professionals to vigilantly monitor patient wellbeing during and after treatment. A comprehensive **[Table: Research Outcomes]** will detail clinical efficacy, safety statistics, and patient-reported outcomes to provide greater insights into Capnat’s performance as a cancer therapeutic. For details on the latest capecitabine research, the UK cancer studies offer valuable context surrounding this drug and its applications. The promising results position Capnat as an increasingly vital player in oncology treatment protocols.
Clinical Effectiveness in the UK
The integration of Capnat into NHS cancer protocols marks a significant advancement in the treatment landscape, especially for patients who have not responded to prior therapies. Analyses of treatment outcomes suggest that Capnat markedly enhances patients' quality of life—many report reduced symptoms commonly associated with cancer treatment. Regular follow-ups have indicated that patient compliance is notably high, largely attributed to access via NHS prescriptions and robust patient education programmes. However, while the integration of Capnat has benefitted many, challenges remain. Issues surrounding the management of side effects and ensuring equitable access across different UK regions are concerns that require ongoing attention. Evidence from patient forums highlights that educational support can play a crucial role in improving adherence to the Capnat treatment regimen, empowering patients with the knowledge needed to navigate their therapies effectively. The NHS continues to adapt its strategies to ensure that Capnat remains a cornerstone in the fight against cancer, with a keen focus on patient-centric care and optimal health outcomes.
Indications & Expanded Uses
Capnat has garnered approval from the MHRA for the treatment of a range of cancers, notably metastatic breast and colorectal cancer. It is primarily utilised for patients who have shown no response to prior treatments. The common treatment regimen followed involves administering 1250 mg/m² twice daily for a duration of 14 days. This ensures that patients receive a robust and targeted approach to their therapy. In addition to its approved uses, oncologists are increasingly exploring off-label applications of Capnat for other solid tumours, thereby capitalising on its effectiveness as a pyrimidine analogue. During patient consultations, healthcare providers often discuss the rationale behind these off-label applications, ensuring informed decision-making. This reflects an evolving landscape in treatment protocols that fuels a propensity for innovative, patient-centred care methodologies in oncology. As the medical community continues to expand its understanding of Capnat’s indications, the role of this medication solidifies within modern cancer therapies, paving the way for more versatile use among oncological practices.
Composition & Brand Landscape
Capnat contains the active ingredient capecitabine, known under the ATC classification L01BC06. This drug is primarily manufactured by Natco Pharma Ltd., available in 500 mg tablet form, packaged in convenient packs of 10. This accessibility makes it easier for patients requiring treatment.
Within the UK, Capnat stands among several brands inclusive of Capecitabine Accord and the original brand, Xeloda. Each of these variants has its unique positioning regarding price and patient preference.
Patients often regard Capnat as a cost-effective alternative, which can be a crucial factor in treatment decisions. This landscape highlights the importance of considering multiple options while ensuring that financial implications don’t overshadow medical needs.
Contraindications & Special Precautions
Capnat is not suitable for everyone. Specific contraindications include patients with known hypersensitivity to capecitabine or any of its components, those suffering from severe renal impairment (creatinine clearance under 30 mL/min), and individuals with dihydropyrimidine dehydrogenase (DPD) deficiency.
Extra caution should be exercised for elderly individuals, patients with cardiac issues, or significant comorbidities. It's vital for healthcare providers to review comprehensive patient histories alongside current medications to avoid adverse interactions. For example:
- Alcohol consumption can intensify side effects.
- Driving may be restricted when experiencing fatigue or dizziness.
Moreover, patient education is key, particularly concerning those receiving anticoagulant therapy, as Capnat can heighten bleeding risks. Understanding and adhering to these precautions ensures a safer treatment experience.
Dosage Guidelines
The standard dosage regimen for Capnat in treating metastatic breast cancer is 1250 mg/m² taken twice daily for 14 days, followed by a 7-day break. Colorectal cancer treatments follow a similar guideline, adjusted based on individual responses and tolerability.
Adjustments are particularly necessary for special populations. For elderly patients over the age of 65, closer monitoring is required, while renal impairment necessitates modifications based on creatinine clearance:
- 30-50 mL/min: Dose reduced to 75%
- Less than 30 mL/min: Not recommended
Generally, children are not prescribed Capnat due to limited safety data, posing a significant risk if prescribed. To maintain dosage regularity, clear guidelines advise patients on missed doses: skip the missed dose and do not double up on subsequent doses. The NHS reinforces these protocols through printed instructions accompanying prescriptions to encourage adherence.
Interactions Overview
Capnat has several significant interactions with food and other medications that healthcare providers must monitor closely. Patients may often wonder how these interactions could impact their treatment. Here are some key points:
- Alcohol: Intensifies nausea and gastrointestinal upset when consuming Capnat.
- Caffeine: High-caffeine drinks, such as coffee and certain teas, can hinder the absorption of Capnat.
- Anticoagulants: There have been reports of interactions, particularly with blood thinners, which may increase the risk of bleeding.
To address such interactions effectively, the MHRA Yellow Card system is in operation to report and evaluate any occurrences. Patients are strongly advised to consult their healthcare providers before starting any new medications or supplements. This consultation ensures a holistic view of their treatment plan, helping to minimise any potential risks associated with Capnat interactions.
Cultural Perceptions & Patient Habits
In the UK, cultural attitudes towards medications like Capnat reflect a mixture of trust and caution. Many patients turn to local pharmacies for advice, often shopping at well-known chains like Boots and LloydsPharmacy. Trust in pharmacists is pivotal and significantly influences adherence to treatment protocols and patients’ perceptions of medication value.
Online patient forums such as Patient.info and Mumsnet indicate that people actively seek pharmacist counselling before making treatment decisions. The role of NHS 111 services further supports this by offering guidance on treatment options and side effects, thereby creating a well-informed patient community.
Social media platforms contribute to this culture by fostering discussions around apprehensions and side effects of Capnat. These platforms serve as community-driven support systems. The blend of professional advice and social engagement showcases a resilient healthcare culture in the UK, making Capnat a common choice among patients after thorough consultations.
Availability & Pricing Patterns
Capnat is readily available in various UK pharmacies, including Boots, LloydsPharmacy, and Superdrug. Patients typically access Capnat through NHS prescriptions, which are charged at a standard rate. However, costs can vary according to specific regional pricing policies between England, Scotland, Wales, and Northern Ireland.
While the NHS covers much of the cost, private purchasing can result in higher expenses, prompting a demand for accessible generics like Capnat. Online pharmacies are becoming more popular, particularly among younger demographics, thanks to the convenience of obtaining medication without a pharmacist's consultation. However, the NHS's electronic prescriptions system helps ensure rigorous monitoring of prescription drugs, promoting patient safety and reducing the risk of misuse.
Delivery Information
| City | Region | Delivery Time |
|---|---|---|
| London | Greater London | 5–7 days |
| Birmingham | West Midlands | 5–7 days |
| Manchester | North West | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Newcastle | North East | 5–7 days |
| Bristol | South West | 5–7 days |
| Sheffield | Yorkshire | 5–7 days |
| Leeds | Yorkshire | 5–7 days |
| Cardiff | Wales | 5–9 days |
| Coventry | West Midlands | 5–9 days |
| Nottingham | East Midlands | 5–9 days |
| Plymouth | South West | 5–9 days |
| Stoke-on-Trent | West Midlands | 5–9 days |
| Derby | East Midlands | 5–9 days |