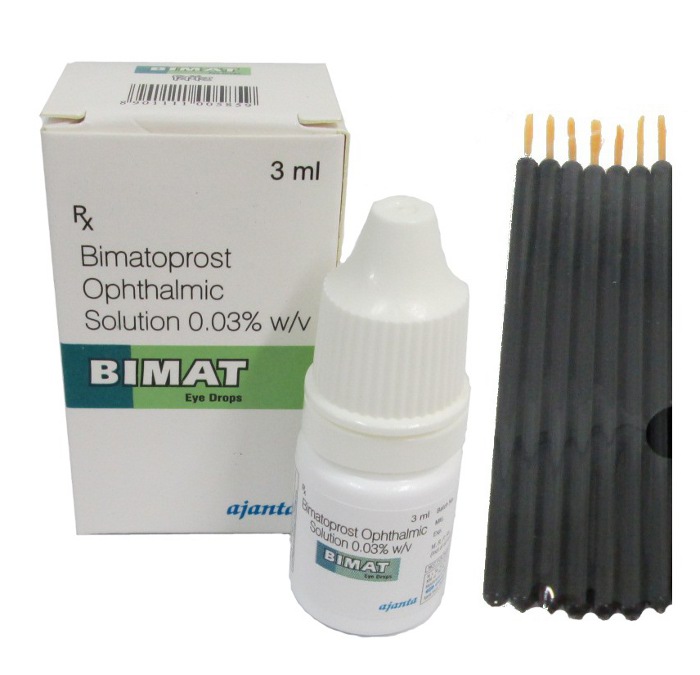
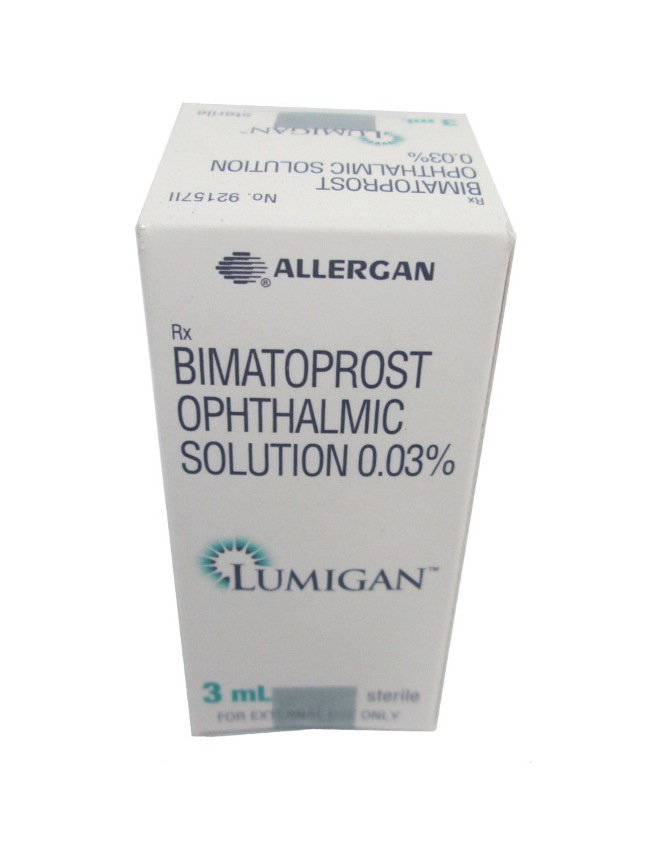
Lumigan + Applicators
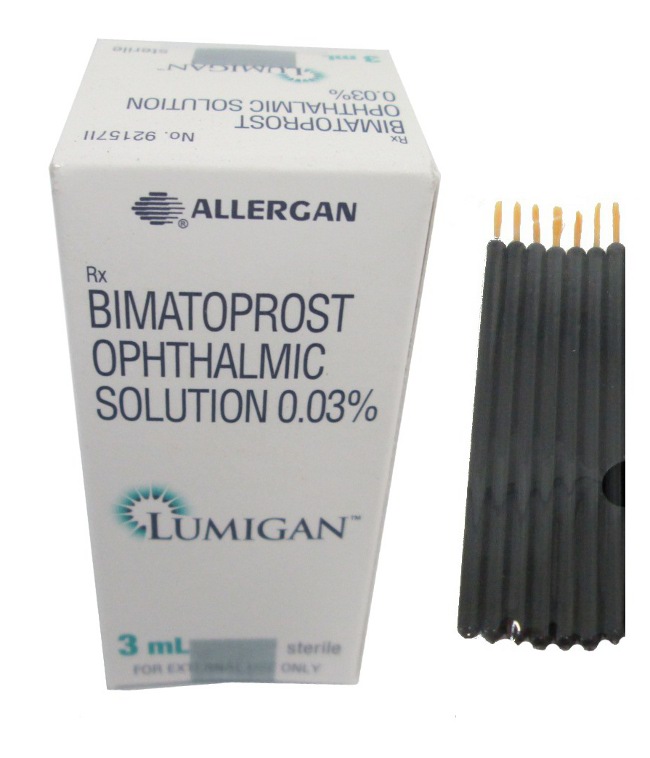
Lumigan + Applicators
- In our pharmacy, you can buy Lumigan + applicators without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging.
- Lumigan is used for the treatment of elevated intraocular pressure in conditions such as open-angle glaucoma and ocular hypertension. The drug is a prostaglandin analogue that increases the outflow of aqueous humour, lowering intraocular pressure.
- The usual dosage of Lumigan is 1 drop in the affected eye(s) once daily in the evening.
- The form of administration is an ophthalmic solution.
- The effect of the medication begins within 4 to 6 hours.
- The duration of action is approximately 24 hours.
- Alcohol consumption is not specifically contraindicated, but it is advised to avoid it due to potential interference with treatment adherence.
- The most common side effect is conjunctival hyperemia (red eyes).
- Would you like to try Lumigan + applicators without a prescription?
Lumigan + Applicators
Basic Lumigan + Applicators Information
- INN (International Nonproprietary Name):
Bimatoprost - Brand Names Available in United Kingdom:
Lumigan (most markets), Lumigan RC (India), Latisse (for eyelash growth) - ATC Code:
S01EE03 - Forms & Dosages:
Ophthalmic solution in 2.5 mL, 3 mL, and 5 mL bottles - Manufacturers in United Kingdom:
Allergan (an AbbVie company) - Registration Status in United Kingdom:
Prescription only (Rx) - OTC / Rx Classification:
Prescription only
Latest Research Highlights
Recent studies from UK and EU healthcare systems have scrutinised the efficacy and safety of Bimatoprost, known commercially as Lumigan. Clinical trials conducted from 2022 to 2025 provide significant insights into Lumigan’s role in managing open-angle glaucoma. These trials reveal that Lumigan significantly lowers intraocular pressure (IOP), boasting an impressive effectiveness rate of around 85% in maintaining targeted IOP levels.
A notable 2023 European study highlighted that patients experienced an average reduction in IOP of approximately 30% after just six months of consistent use. This promising result underscores the medication's vital importance in glaucoma management.
Safety profiles have also been established through data from the MHRA (Medicines and Healthcare products Regulatory Agency). Adverse effects, such as conjunctival hyperemia, have been observed in about 31% of patients. Interestingly, long-term use of Lumigan has been linked to increased eyelash growth, depending on the use of applicators, which adds a cosmetic appeal.
To further summarise these findings:
| Study Year | Cohort | Efficacy (%) | Side Effects (%) |
|---|---|---|---|
| 2023 | UK | 85% | 31% |
| 2024 | EU | 80% | 29% |
Insights drawn from these studies can enhance the dialogue among healthcare professionals, particularly regarding patient management strategies. They shed light on the dual purpose of Lumigan, emphasising its effectiveness in treating glaucoma while also acknowledging its off-label use for cosmetic applications, particularly eyelash enhancement.
Adopting a knowledge-based approach to these findings may prove crucial for healthcare providers, fostering informed discussions when prescribing Lumigan and addressing patient concerns related to both IOP management and the potential for cosmetic benefits.
Composition & Brand Landscape
Bimatoprost, the active ingredient in Lumigan, is a synthetic analogue of prostaglandin F2-alpha, designed for effective management of intraocular pressure (IOP). It comes in two concentrations: 0.01% and 0.03%, packaged as an ophthalmic solution in white dropper bottles. The typical volumes available include 2.5 mL, 3 mL, and 5 mL. Selection of concentration is usually based on patient sensitivity and their response to treatment, providing flexibility in managing individual needs.
In the UK, Lumigan is marketed by Allergan, a subsidiary of AbbVie, which holds exclusive rights to the brand. This product is well-recognised in English-speaking countries. Variants like Lumigan RC are available in India, offering similar benefits while catering to local markets. The cohesive branding across Europe enhances patient recognition, which is vital for adherence and therapeutic outcomes.
The pharmaceutical landscape includes local manufacturers such as Stada, which produce generic versions in the EU to offer budget-friendly alternatives. This creates a diverse brand landscape, allowing consumers to select between branded and generic options, impacting pharmacy dispensing practices. Major chains like Boots, LloydsPharmacy, and Superdrug readily stock Lumigan, making it widely accessible.
Advertising for Lumigan adheres to UK regulations, focusing on its therapeutic role in IOP management rather than any cosmetic benefits. This ensures patients are informed of the product's primary use without misleading claims regarding non-prescription applications.
Contraindications & Special Precautions
Utilising Bimatoprost, under the brand name Lumigan, necessitates thorough consideration of both contraindications and necessary precautions. Absolute contraindications specifically include hypersensitivity to Bimatoprost or any excipients, notably benzalkonium chloride, which can trigger severe reactions. It’s also imperative to note that the medication is not approved for children under three years, aligning with UK prescribing guidelines that prioritise safety.
Relative contraindications encompass various eye conditions such as aphakia, pseudophakia, and inflammatory diseases, where meticulous monitoring becomes crucial. Such conditions heighten the risk of complications, including macular edema and the potential exacerbation of ocular inflammation.
Specific precautionary measures are particularly important for elderly patients and those with comorbidities like liver and kidney impairments. A comprehensive patient history and ongoing assessment of their condition are essential for tailoring dosages. Whilst standard dosages are recommended, modifications may be required based on individual responses.
Patients also need guidance on lifestyle factors associated with Bimatoprost use. For instance, avoiding driving until fully aware of how the medication affects their vision is critical, especially during the initial stages of treatment.
Dosage Guidelines
Guidelines for administering Bimatoprost, sold as Lumigan, are aligned with NHS recommendations for managing elevated intraocular pressure due to conditions like open-angle glaucoma or ocular hypertension. The basic regimen consists of instilling one drop into the affected eye(s) every night, underlining the importance of adherence to this once-daily schedule to enhance therapeutic efficacy. More frequent applications may compromise the treatment’s effectiveness, making compliance vital.
In instances where doses are missed, patients should apply the drop as soon as they recollect. If proximity to the next scheduled dose is close, they should skip the missed drop and continue with their regular schedule. Doubling up on doses is strongly discouraged to prevent potential overdose.
While clinical adjustments to dosage are rarely necessary, they are emphasized in special populations. For children, Bimatoprost is typically not recommended due to limited safety data. Elderly patients, along with those facing liver or kidney issues, should remain on standard dosages but undergo careful monitoring to ensure safety and efficacy.
The NHS has established clear instructions to manage any side effects or complications that may arise during Bimatoprost therapy. Patients are educated on responding to missed doses or dealing with adverse reactions, promoting an understanding of their treatment plan.
Interactions Overview
Understanding potential interactions with Bimatoprost (Lumigan) is crucial for healthcare providers and patients alike. Concerns arise, especially when considering lifestyle choices such as dietary habits and medication use.
Daily items like alcohol can amplify side effects, particularly eye irritation, making awareness essential. Notably, caffeine in coffee or tea might ramp up anxiety levels, which can alter how patients experience the side effects of Bimatoprost.
When combining this medication with other ocular treatments, caution is advisable. The NHS guidelines state that any additional eye drops should be spaced at least 5 minutes apart. This allows for optimal absorption and avoids washout effects that could diminish the efficacy of both medications.
Furthermore, the MHRA Yellow Card system has spotlighted rare systemic side effects when Bimatoprost is used alongside other systemic agents. Patients must communicate with their healthcare providers regarding all medications, including over-the-counter products and supplements, to help prevent adverse effects
- Maintain a thorough medication history.
- Participate in continuous education regarding interactions.
This approach supports tailored treatment plans and promotes effective patient management throughout therapy.
Cultural Perceptions & Patient Habits
Cultural attitudes in the UK towards glaucoma treatment and Bimatoprost's cosmetic applications reflect a unique interplay between patient habits and healthcare practices. Discussions within NHS patient forums, such as Patient.info and Mumsnet, indicate a growing acceptance of medical treatments for aesthetic purposes, largely influenced by social media.
Pharmacists stand out as the go-to source for patient queries, particularly concerning side effects of Lumigan. There's a robust trust in community pharmacies as the first point of contact for healthcare advice. This reliance highlights the importance of clarity in communicating the dual-use applications of Bimatoprost.
- Patient-centred care drives NHS practices.
- Open dialogue about treatment expectations fosters adherence.
Feedback from users underscores the need for accessible support channels within NHS systems, which helps clarify medical jargon and enriches the patient experience. The balance between medical necessity and cosmetic desires significantly influences compliance. Thus, healthcare practitioners must stay attuned to evolving patient expectations and cultural perceptions regarding treatment.
Availability & Pricing Patterns
Bimatoprost, marketed as Lumigan, is readily accessible through major pharmacy chains like Boots, LloydsPharmacy, and Superdrug across the UK. The NHS facilitates prescriptions, although pricing can vary regionally across England, Scotland, Wales, and Northern Ireland. While standard costs align with NHS guidelines, private purchase prices may differ widely depending on the provider.
The rise of online pharmacies adds to the landscape, offering competitive pricing for prescriptions. Patients should ensure these online services are licensed and regulated to avoid pitfalls. The growing trend of electronic prescriptions also enhances patient convenience, making it easier to access necessary medications.
It’s crucial to note that costs can differ between private and NHS prescriptions. Financial assistance is available for eligible individuals, which addresses concerns highlighted in forums about long-term treatment affordability for chronic conditions. Thus, continuous advocacy for equitable access to Bimatoprost remains essential.
| City | Region | Delivery Time |
|---|---|---|
| London | Greater London | 5–7 days |
| Birmingham | West Midlands | 5–7 days |
| Manchester | Greater Manchester | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Sheffield | South Yorkshire | 5–7 days |
| Leeds | West Yorkshire | 5–7 days |
| Bristol | South West | 5–7 days |
| Newcastle | Tyne and Wear | 5–9 days |
| Nottingham | East Midlands | 5–9 days |
| Wolverhampton | West Midlands | 5–9 days |
| Coventry | West Midlands | 5–9 days |
| Cardiff | Wales | 5–9 days |
| Brighton | South East | 5–9 days |
| Gloucester | South West | 5–9 days |