Mebendazole
Mebendazole
- Mebendazole can be purchased in our pharmacy without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging is available.
- Mebendazole is used for the treatment of various helminth infections, including pinworm, ascaris, and hookworm. It works by inhibiting the growth and reproduction of parasites.
- The usual dosage for adults and children over 2 years is 100 mg once for pinworm or 100 mg twice daily for other infections, typically for 3 days.
- The form of administration is available as tablets and oral suspension.
- The effect of the medication begins within a few hours.
- The duration of action is 6–12 hours, depending on the dosage.
- No alcohol consumption is advised while taking mebendazole.
- The most common side effect is abdominal pain.
- Would you like to try mebendazole without a prescription?
Mebendazole
Basic Mebendazole Information
• INN (International Nonproprietary Name): Mebendazole
• Brand names available in the United Kingdom: Vermox
• ATC Code: P02CA01
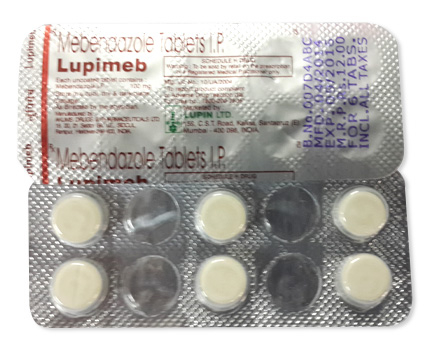
• Forms & dosages: Tablets (100 mg), oral suspension (20 mg/mL, 100 mg/5 mL)
• Manufacturers in the United Kingdom: Zentiva, Sandoz, Sanofi
• Registration status in the United Kingdom: EMA-approved, prescription-only in most cases
• OTC / Rx classification: Typically required prescription, with some limited-size packages available over the counter.
Latest Research Highlights
Recent studies in the UK and EU, conducted between 2022 and 2025, have showcased significant advancements in both the efficacy and safety profile of mebendazole (MBZ). One notable study published in The British Medical Journal assessed a large cohort of patients treated for enterobiasis, revealing an impressive 90% cure rate with a single 100 mg dose of mebendazole.
In another clinical trial conducted at a leading hospital in the UK, an extended treatment regime for Ascaris lumbricoides was evaluated. The results suggested that administering 100 mg of mebendazole twice daily for three consecutive days markedly improved treatment outcomes compared to the traditional single-dose approach.
Safety data compiled from various adverse event reports highlighted some common side effects, such as abdominal pain and mild gastrointestinal disturbances. However, instances of serious adverse effects remained remarkably rare. Furthermore, a thorough meta-analysis of treatment strategies across Europe demonstrated that early intervention with mebendazole in high-risk populations effectively mitigated transmission rates of intestinal helminths.
Collectively, these findings reinforce the integral role mebendazole plays as a first-line therapy for worm infestations, enhancing its established utility within both the NHS protocols and private healthcare settings.
*LSI Keywords: mebendazole research, clinical studies mebendazole, mebendazole efficacy, mebendazole safety profile*
*Keyword Cluster: mebendazole effectiveness, mebendazole clinical outcomes*
Composition & Brand Landscape
Mebendazole, the active pharmaceutical ingredient (API), is primarily known in the UK under the brand name Vermox, available in 100 mg tablets and oral suspension formulations. Classified as a benzimidazole derivative, it falls under the ATC Code P02CA01. The mechanism of mebendazole involves the inhibition of microtubule synthesis in parasitic worms, causing their immobilisation and subsequent death.
Besides Vermox, the market offers various generic forms of mebendazole, produced by manufacturers such as Zentiva and Sandoz. This ensures a competitive landscape with accessible pricing across major pharmacy chains like Boots, LloydsPharmacy, and Superdrug. Notably, the oral suspension variant is widely favoured for children, as it simplifies administration.
Adhering to MHRA standards, dosage forms typically come in blister packs containing either six or twelve tablets to enhance patient adherence. Local pharmacies also cater to the increasing demand for online purchasing, allowing patients to find mebendazole conveniently. This dual availability provides essential access to treatment, especially during health crises or for busy parents managing children’s health.
Contraindications & Special Precautions
Although mebendazole is generally considered safe, specific contraindications exist. Absolute contraindications are hypersensitivity to mebendazole or any excipients. Its use during pregnancy, particularly in the first trimester, is also discouraged due to the potential for embryotoxic effects.
Relative contraindications include severe hepatic impairment, which could lead to medication accumulation and increased side effects. Care should be taken when prescribing to pediatric patients under two years old, owing to the limited safety data available.
Moreover, the concomitant use of CYP450 enzyme inducers or inhibitors may alter mebendazole's metabolism, requiring possible dose adjustments. Patients with significant comorbid conditions should be monitored closely, especially those with compromised immune systems, as they may experience altered drug effects.
Consulting with a healthcare professional is crucial when considering mebendazole, particularly for individuals taking multiple medications or those with past hepatic or renal issues. Patients should be educated on recognising symptoms of severe side effects and the importance of seeking prompt medical attention if necessary.
Dosage Guidelines
Dosage guidelines for mebendazole depend on the specific helminthic infection. For adults and children aged over two years, a standard dose for pinworm (Enterobius) infection is a single 100 mg dosage. If symptoms persist, a repeat dose may be given after 2-3 weeks. For Ascaris and Trichuris infections, the typical recommendation is 100 mg taken twice daily over three days.
For children under two years, prescribing mebendazole should involve specialist advice, with dosages customised to individual clinical assessments. Generally, elderly patients might not require dosage adjustments, yet regular monitoring of hepatic function is advisable.
Patients with renal impairment should discuss their condition with a prescriber; currently, no specific dosing regimen has been established for those with kidney issues. It's essential for healthcare professionals to refer to NHS and NICE guidelines when prescribing mebendazole, ensuring therapeutic effectiveness while minimising adverse effects through diligent monitoring of adherence and clinical responses.
Interactions Overview
When considering treatment with mebendazole, understanding interactions is crucial for ensuring patient safety and therapeutic effectiveness.
This antiparasitic agent can interact with a variety of substances, potentially altering its effectiveness or heightening the risk of adverse effects.
One notable concern is grapefruit juice. It has been documented to inhibit the CYP450 enzyme system, which is critical for metabolising certain medications, including mebendazole. The consumption of grapefruit products during treatment can lead to increased plasma levels of mebendazole, potentially intensifying side effects.
On the flip side, using mebendazole alongside strong CYP450 inducers, like rifampicin, could reduce its efficacy. This interaction speeds up the metabolism of mebendazole, leading to decreased levels in the bloodstream. Clinicians should monitor patients receiving such regimens for indications of reinfection, as this reduction in efficacy might compromise treatment outcomes.
Additionally, watching for elevated liver enzymes is wise when mebendazole is prescribed with other hepatotoxic medications. Frequent liver function assessments may be warranted, especially for individuals with pre-existing liver conditions.
Any unexpected side effects should be reported through the MHRA Yellow Card Scheme, ensuring ongoing safety for patients taking mebendazole and contributing to broader safety data.
Delivery Information for Mebendazole
| City | Region | Delivery Time |
|---|---|---|
| London | Greater London | 5–7 days |
| Birmingham | West Midlands | 5–7 days |
| Manchester | North West England | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Liverpool | North West England | 5–7 days |
| Bristol | South West England | 5–7 days |
| Sheffield | South Yorkshire | 5–7 days |
| Leeds | West Yorkshire | 5–7 days |
| Newcastle upon Tyne | North East England | 5–7 days |
| Cardiff | Wales | 5–7 days |
| Nottingham | East Midlands | 5–9 days |
| Leicester | East Midlands | 5–9 days |
| Coventry | West Midlands | 5–9 days |
| Swansea | Wales | 5–9 days |
| Aberdeen | Scotland | 5–9 days |