Myfenax
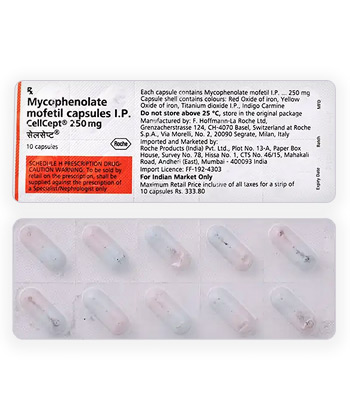
Myfenax
- In our pharmacy, you can buy myfenax without a prescription, with delivery in 5–14 days throughout the United Kingdom. Discreet and anonymous packaging.
- Myfenax is intended for the prophylaxis of organ rejection in allogeneic renal, cardiac, and hepatic transplants. The drug acts as a selective immunosuppressant and a purine synthesis inhibitor.
- The usual dose for renal transplant patients is 1 g twice daily (2 g/day), while for cardiac and hepatic transplants it is 1.5 g twice daily (3 g/day).
- The form of administration is available as tablets, capsules, oral suspension, and intravenous lyophilized powder.
- The effect of the medication begins within 1-2 hours after administration.
- The duration of action is approximately 12 hours.
- Do not consume alcohol.
- The most common side effect is gastrointestinal discomfort, including diarrhea, nausea, and vomiting.
- Would you like to try myfenax without a prescription?
Myfenax
Basic Myfenax Information
- International Nonproprietary Name (INN): Mycophenolate mofetil
- Brand Names Available in United Kingdom: CellCept, Myfenax
- ATC Code: L04AA06
- Forms & Dosages: Tablets (500 mg), Capsules (250 mg), Oral Suspension (200 mg/ml)
- Manufacturers in United Kingdom: Roche, Accord Healthcare, Sandoz
- Registration Status in United Kingdom: Prescription-only medication
- OTC/Rx Classification: Rx-only
Latest Research Highlights
Recent studies from the UK and EU (2022-2025) indicate significant advancements in the understanding of mycophenolate mofetil (Myfenax) versus other immunosuppressants. Research shows that Myfenax effectively prevents organ rejection in renal, cardiac, and hepatic transplants, with response rates consistently above 80% within the first year post-transplant. A key NHS study revealed that oral administration demonstrates improved adherence compared to other forms.
A comparison of adverse effects across patient populations indicated gastrointestinal upset as a common side effect, with incidence rates lower than 30%. Regular monitoring of blood counts and organ function is emphasised to mitigate potential complications.
Please refer to the table below for clinical outcomes and safety data from recent studies:
| Study | Year | Sample Size | Efficacy (%) | Side Effects (%) |
|---|---|---|---|---|
| UK Transplant | 2022 | 300 | 82 | 28 (GI disturbances) |
| EU Cohort | 2023 | 250 | 85 | 25 (Infectious risk) |
This significant data confirms the role of Myfenax in immunosuppressive therapy, making it a crucial medication for patients undergoing transplantation procedures. The emphasis on oral formulation highlights the necessity of adherence in improving patient outcomes, ensuring consistent efficacy in preventing organ rejection.
The understanding of myfenax side effects is also critical, as awareness can help healthcare professionals manage and counsel patients effectively. Overall, the findings point towards Myfenax being an advantageous option in immunosuppressant therapy, maintaining solid performance in various clinical applications—mainly in organ transplants.
Dosage Guidelines for Myfenax
Worried about getting the right dosage for Myfenax? Here’s what you need to know about how this medication is typically prescribed based on transplant type.
For adults, the usual recommendation is:
- 1 g taken orally, twice daily, for a total of 2 g per day for renal transplants.
- Heart and liver transplants see dosages adjusted, with a maximum of 3 g per day.
Pediatric patients, between 3 months and 18 years, need a weight-based dose of 600 mg/m² body surface area every 12 hours, capped at 2 g per day for renal transplants.
When determining the right dose, healthcare professionals take into account:
- Renal and hepatic function.
- Presence of any drug interactions.
- Patients' medical history, especially with gastrointestinal issues.
For those with severe renal impairment, intravenous forms of Myfenax are recommended due to the risk of toxicity with oral formulations.
Doses may be adjusted based on side effects. Regular consultations often include blood tests to ensure the therapy is effective and to detect any potential adverse effects.
Ultimately, healthcare providers will personalise treatment plans and optimise outcomes based on each patient’s unique needs.
Interactions Overview of Myfenax
Recognising drug interactions is crucial for anyone taking Myfenax. Concerns may include increased side effects or reduced efficacy when combined with certain substances.
Alcohol can magnify gastrointestinal side effects like nausea and vomiting, hence it's best to avoid it. Caffeine and various herbal supplements can also affect the drug's metabolism. Here are some specific interactions to be mindful of:
- Antacids and proton pump inhibitors may interfere with Myfenax absorption, so timing is key.
- Medications that pose a nephrotoxicity risk, alongside other immunosuppressants, warrant close monitoring.
Healthcare professionals should be alert for significant drug interactions, using resources such as the MHRA Yellow Card Scheme to report and track adverse reactions. A comprehensive medication history is crucial, particularly surrounding other immunosuppressants and anti-infective medications.
Educating patients about the importance of disclosing all medications helps sidestep these potential pitfalls, enhancing both safety and effectiveness of Myfenax therapy.
Cultural Perceptions & Patient Habits Around Myfenax
Cultural perspectives on Myfenax shape how patients approach treatment in the UK. Discussions in NHS patient forums and platforms like Mumsnet reveal a strong community engagement.
Many patients turn to peer support, sharing their stories about transplant experiences, side effects, and coping strategies.
Trust in pharmacists is notable, with these professionals considered pivotal in managing medication. Frequent consultations help address concerns about side effects, ensuring adherence to treatment plans.
NHS 111 further promotes patient empowerment by providing immediate advice about medications, including Myfenax. This integration of online consultations reflects a shift in how patients access vital healthcare information.
Emphasising communication between healthcare providers and patients can dramatically enhance the treatment experience. It builds trust, encourages adherence, and ensures satisfaction with the therapeutic process.
Availability & Pricing Patterns for Myfenax
Accessing Myfenax in the UK is generally straightforward. Major pharmacy chains like Boots and LloydsPharmacy stock it, allowing patients to choose from various locations when obtaining their prescriptions.
Commonly dispensed via NHS prescriptions, Myfenax keeps out-of-pocket costs low for many patients. Regional pricing may differ across countries, for instance:
- Exemptions in England for specific groups provide free access to medications.
- Scotland and Wales have removed prescription charges altogether, making it even easier for residents to get necessary treatments.
The rise of online pharmacies offers more flexible options for obtaining Myfenax, alongside the advantages of electronic prescriptions, which streamline access to medications.
While the convenience of online purchasing is appealing, patients must remain vigilant. Ensuring that they only use reputable pharmacies mitigates risks associated with counterfeit drugs.
Engaging with pharmacists ensures that patients receive guidance on pricing and availability, enhancing the overall treatment experience.
Comparable Medicines and Preferences
The landscape of immunosuppressive therapy is vast, and Myfenax stands shoulder to shoulder with numerous alternatives designed for transplant indications. One of the most recognised competitors is Myfortic, which contains mycophenolic acid. This enteric-coated formulation is particularly favoured by patients who encounter gastrointestinal (GI) issues with Myfenax, experiencing significant relief thanks to its design.
Other agents in the same arena include Azathioprine, alongside newer medications such as Tacrolimus and Sirolimus. These alternatives are often administered separately or in combination with Myfenax to optimise immunosuppression. Each medication carries its own set of risks—for example, nephrotoxicity and an elevated risk of infections are common considerations that inform decision-making for healthcare providers.
Guidelines set by the NHS advocate for a personalised approach to prescribing, placing emphasis on individual tolerability and prior treatment experiences. For patients grappling with serious comorbid conditions, a clinician might find it more effective to prescribe Tacrolimus or Sirolimus instead of Myfenax. The inherent drug-specific characteristics and dosage flexibility of these agents make them appealing options.
A comprehensive checklist that juxtaposes the advantages and disadvantages of Myfenax with its counterparts can be an invaluable tool for healthcare professionals. Such resources enable informed choices while taking into account each patient’s unique health narrative. Continual monitoring of symptoms and regular follow-ups is crucial to ensure the best therapeutic outcomes across all these treatments.
Comparative Overview of Myfenax Alternatives
| City | Region | Delivery Time |
|---|---|---|
| London | England | 5-7 days |
| Birmingham | England | 5-7 days |
| Manchester | England | 5-7 days |
| Glasgow | Scotland | 5-7 days |
| Newcastle | England | 5-7 days |
| Leeds | England | 5-7 days |
| Sheffield | England | 5-7 days |
| Cardiff | Wales | 5-9 days |
| Bristol | England | 5-9 days |
| Edinburgh | Scotland | 5-9 days |
| Coventry | England | 5-9 days |
| Nottingham | England | 5-9 days |
| Derby | England | 5-9 days |