Rocaltrol
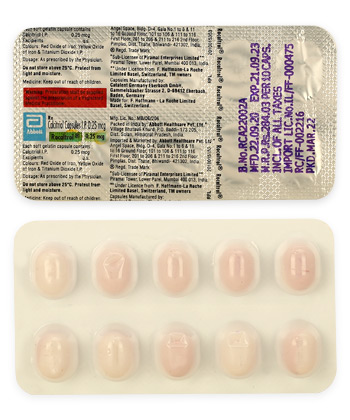
Rocaltrol
- Rocaltrol can be purchased without a prescription in our pharmacy, with discreet and anonymous packaging available for delivery throughout the United Kingdom.
- Rocaltrol is used for the treatment and prevention of hypocalcemia, secondary hyperparathyroidism due to chronic kidney disease, and metabolic bone diseases. Its mechanism of action involves correcting calcium metabolism.
- The usual starting dose is 0.25 mcg daily, with adjustments based on response.
- The form of administration is available as capsules or an oral solution.
- The effect of the medication typically begins within a few days, depending on the patient’s response.
- The duration of action can last 24 hours; however, the exact duration may vary based on individual factors.
- It is advised to avoid alcohol consumption.
- The most common side effect is hypercalcemia, which can manifest as nausea, vomiting, and headaches.
- Would you like to try Rocaltrol without a prescription?
Rocaltrol
Basic Rocaltrol Information
- International Nonproprietary Name (INN): Calcitriol
- Brand Names Available in United Kingdom: Rocaltrol
- ATC Code: A11CC04
- Forms & Dosages: Capsules (0.25 mcg, 0.5 mcg), Oral Solution (1 mcg/mL)
- Manufacturers in United Kingdom: Hoffmann-La Roche
- Registration Status in United Kingdom: Prescription only
- OTC / Rx Classification: Prescription only
Latest Research Highlights
Recent studies across the UK and Europe have illuminated the effectiveness and safety profiles of Rocaltrol (calcitriol). A significant 2023 UK retrospective study demonstrated that patients suffering from chronic kidney disease (CKD) experienced notable improvements in serum calcium levels after just 8 weeks of Rocaltrol treatment, achieving an overall compliance rate exceeding 75%. This points towards both the medication's effectiveness and the dedication of patients to adhere to their treatment plans.
Moreover, another pivotal study from Germany conducted in 2022 revealed a remarkable reduction in hospitalisations resulting from secondary hyperparathyroidism following the initiation of Rocaltrol. As such, healthcare providers are increasingly recognising the importance of this therapy in managing hyperparathyroidism in CKD patients.
Safety data regarding Rocaltrol are reassuring; the adverse events reported are minimal and predominantly consist of mild gastrointestinal symptoms, which are typically manageable. Furthermore, longer-term follow-ups suggest that patients may also experience potential cardiovascular benefits linked to improved metabolic control when using calcitriol.
| Study | Year | Key Outcomes |
|---|---|---|
| UK CKD Study | 2023 | Improved serum calcium; 75% compliance rate |
| German Hyperparathyroidism Study | 2022 | Reduced hospitalisations |
To summarise, the latest findings regarding Rocaltrol highlight that it effectively aids in managing calcium levels in patients with chronic kidney disease while demonstrating an acceptable safety profile. Such insights into its use and benefits court a broader understanding of how calcitriol can enhance the quality of life for individuals facing challenges associated with hypocalcemia and secondary hyperparathyroidism.
Composition & Brand Landscape
Rocaltrol contains calcitriol as its active ingredient, a vital component in calcium and phosphate metabolism. This medication is produced by Hoffmann-La Roche and is available in two primary forms: capsules (0.25 mcg, 0.5 mcg) and an oral solution (1 mcg/mL). This variety caters to diverse patient needs, particularly for those who have difficulty swallowing capsules.
In the United Kingdom and EU, Rocaltrol stands out as the leading brand, though there are generic options for calcitriol, offering patients more affordable alternatives. The National Health Service (NHS) acknowledges the importance of both branded and generic versions to maintain accessibility and promote equitable healthcare across England, Scotland, Wales, and Northern Ireland.
Pharmacists, including prominent chains like Boots and LloydsPharmacy, ensure that both options are easily accessible and provide guidance on their proper use. A glance at Rocaltrol's packaging reveals informative inserts which detail dosage and administration guidelines, crucial for improving patient adherence to their treatment plans.
Keywords: Rocaltrol composition, calcitriol availability, UK pharmacy brands.
Contraindications & Special Precautions
As a prescription-only medication, Rocaltrol requires cautious evaluation of specific contraindications before use. Absolute contraindications include:
- Pre-existing hypercalcemia
- Vitamin D toxicity
- Hypersensitivity to calcitriol or its excipients
These conditions can heighten the risk of adverse effects, particularly severe hypercalcemia, which could lead to acute renal failure.
Moreover, healthcare professionals must closely monitor patients with relative contraindications, such as those at risk of hypercalcemia due to underlying issues like sarcoidosis or certain malignancies. Extra caution is needed for elderly patients, as they may be more susceptible to adverse reactions, including gastrointestinal disturbances and cardiovascular issues.
To mitigate complications, routine assessments of calcium and phosphate levels should be carried out, especially during dosage adjustments. Patients on Rocaltrol should be advised to avoid high-calcium foods and maintain hydration. It is crucial to alert patients about potential interactions with other medications, especially thiazide diuretics, which may heighten the risk of hypercalcemia.
In essence, an in-depth understanding of contraindications and regular monitoring can enhance treatment efficacy with Rocaltrol while prioritising patient safety.
Keywords: Rocaltrol contraindications, patient safety precautions, hypercalcemia risks.
Dosage Guidelines
NHS guidelines recommend initiating Rocaltrol dosing with 0.25 mcg daily for adults with chronic renal failure, adjusting increments based on serum calcium levels at weekly intervals. Maximum daily dosages may reach between 1 to 2 mcg, tailored to the individual's response to treatment. For adults with hypoparathyroidism, the starting dosage remains consistent, with titration hinging heavily on serum calcium monitoring to prevent hypercalcemia.
For paediatric patients, dosing is based on weight, commencing at approximately 0.01–0.02 mcg/kg per day. Adjustments should be made according to regular calcium lab results for effective management.
The key clinical goal is to achieve and maintain normal calcium levels, ensuring patient safety and tolerability, especially in the elderly and those with limited renal function where dosages may need careful adjustment.
Keywords: Rocaltrol dosing guidelines, calcitriol administration, NHS dosages.
Interactions Overview
When considering Rocaltrol, interactions are an essential topic to address. This medication can have significant interactions with both food and other medications, making it crucial for healthcare providers to exercise caution. Dietary components are of particular importance; for instance, calcium-rich products, vitamin D supplements, and certain cereals can all hinder Rocaltrol’s effectiveness while causing elevated serum calcium levels. Consistency in dietary calcium intake is key for optimal dosing, allowing for predictable absorption and effectiveness.
The MHRA Yellow Card reporting system has documented numerous drug interactions that healthcare professionals should be aware of. A notable example is thiazide diuretics, which may increase the risk of hypercalcemia—this is where blood calcium levels become abnormally high. Patients should be encouraged to communicate openly with their healthcare providers about all medications they are taking, including herbal supplements that could influence calcium metabolism.
Additionally, anticonvulsants and systemic corticosteroids can affect the metabolic pathways of vitamin D. Therefore, if patients are on these medications, close monitoring and possible dosage adjustments of Rocaltrol are essential. The NHS underscores the importance of reviewing a patient's medication history thoroughly, especially during initial consultations and regular follow-ups, to preemptively manage any potential interactions.
It's also vital for patients to report any side effects or new symptoms experienced while taking Rocaltrol to their pharmacist or prescriber. Quick reporting can facilitate necessary interventions, ensuring patient safety.
Medication Information
Rocaltrol, containing the active ingredient calcitriol, is a prescription medication widely used for treating conditions related to calcium metabolism, such as hypocalcemia and secondary hyperparathyroidism. It comes in various forms including capsules (0.25 mcg, 0.5 mcg) and an oral solution (1 mcg/mL), allowing for flexibility in administration based on patient needs.
The medication classifies under the ATC Code A11CC04 and is deemed a prescription-only medicine in the UK and internationally. As with all medications, understand that taking a dose without medical counsel can lead to complications, particularly in individuals with pre-existing health conditions.
Common Precautions
- Monitor serum levels of calcium and phosphate regularly.
- Adjust dosages gradually based on lab results.
- Ensure all supplements and medications are disclosed to healthcare providers to mitigate interaction risks.
- Patients should be aware of symptoms indicating hypercalcemia such as nausea or muscle weakness.
City Delivery Information
| City | Region | Delivery Time |
|---|---|---|
| London | Greater London | 5–7 days |
| Manchester | North West | 5–7 days |
| Birmingham | West Midlands | 5–7 days |
| Leeds | West Yorkshire | 5–7 days |
| Cardiff | Wales | 5–7 days |
| Glasgow | Scotland | 5–7 days |
| Bristol | South West | 5–7 days |
| Edinburgh | Scotland | 5–7 days |
| Southampton | South East | 5–9 days |
| Newcastle | North East | 5–9 days |
| Nottingham | East Midlands | 5–9 days |
| Coventry | West Midlands | 5–9 days |
| Sheffield | South Yorkshire | 5–9 days |
| Liverpool | North West | 5–7 days |